

Symbols Glossary

Symbols Glossary

British Pharmacopoeia
The British Pharmacopoeia (BP) is a major regional pharmacopoeia which provides common quality standards throughout the pharmaceutical industry in Europe to control the quality of medicines, and the substances used to manufacture them.
Reference: The British Pharmacopoeia - British Pharmacopoeia

Caution
Indicates a warning notice in the text.
Reference: BS EN ISO 15223-1:2016 REF No: 5.2.4

CE Mark/CE Mark with Notified Body Number
Indicates comformity with the relevant director or regulation:
- Medical Devices Director EC 93/42/EEC
- Medical Device Regulation 2017/745
Reference: European MDD 93/42/EEC (14 June 1993) - Described in Article 17
European Medical Device Regulation 2017/745 - Described in Article 20

Date of Manufacture
Indicates the date when the medical device was manufactured.
Reference: BS EN ISO 15223-1:2016 REF No: 5.2.6

Do Not Re-Sterilise
Do not re-sterilise the medical device.
Reference: BS EN 1SO 15223-1:2016 REF No: 5.2.6

Do Not Use if Packaging is Damaged
Indicates a medical device should not be used if packaging has been damaged or opened.
Reference: BS EN ISO 15223-1:2016 REF No: 5.3.8

Drug Tariff
Indicates product listing on Drug Tariff.

Authorised Representative in European Community
Indicates the authorised representative within the European Community.
Reference: BS EN ISO 151223-1:2016 REF No: 5.1.2

European Pharmacopoeia
The European Pharmacopoeia (EP) is a major regional pharmacopoeia which provides common quality standards throughout the pharmaceutical industry in Europe to control the quality of medicines, and the substances used to manufacture them.
Reference: European Pharmacopoeia Online (edqm.eu)

Use By Date
Indicates the product expiry date.
Reference: BS EN ISO 15223-1:2016 REF No: 5.1.4

Humidity Limitation
Indicates the range of humidity which the medical device can be safely exposed to.
Reference: BS EN ISO 151223-1:2016 REF No: 5.3.7

Instructions For Use
Consult the medical device's instructions for use.
Reference: BS EN ISO 15223-1:2016 REF No: 5.4.3

Keep Dry
Store product in a dry environment.
Reference: BS EN ISO 15223-1:2016 REF No: 5.4.3

Contains or Presence of Natural Rubber Latex
Manufactured with natural rubber latex.
Reference: BS EN ISO 151223-1:2016 REF No: 5.4.5

Batch Code
Indicates the manufacturer's batch number.
Reference: BS EN ISO 15223-1:2016 REF No: 5.1.5

Manufacturer
Manufacturer - the person placing the device on the market.
Reference: BS EN ISO 15223-1:2016 REF No: 5.1.1

Medical Device
Indicates that the device is a medical device as defined in the MDR 2017/745.
Reference: MDR 2017/745

Non-Sterile
Product is supplied non-sterile.
Reference: BS EN ISO 15223-1:2016 REF No: 5.2.7

Product Number
Indicates the manufacturer's
Reference: BS EN ISO 15223-1:2016 REF No: 5.1.1

Sales Ready Packaging
The product is sold with sales ready packaging.

Prescription Device
Caution - USA Federal Law restricts this device to sale by, or on the order of, physician.
Reference: 21 CFR 801.109

Do Not Re-Use
Product is for single-use only and must not be re-used or re-processed.
Reference: BS EN ISO 15223-1:2016 REF No: 5.4.2

Sterilised by Ethylene Oxide
Product is sterilised using Ethylene Oxide.
Reference: BS EN ISO 15223-1:2016 REF No: 5.2.3

Sterile
Indicates a medical device that has been subjected to a sterilisation process.
Reference: BS EN ISO 15223-1:2016 REF No: 5.2.1

Sterilised by Gamma Radiation
Product is sterilised by Gamma Radiation.
Reference: BS EN ISO 15223-1:2016 REF No: 5.2.4

UKCA
New UK product marking that is required for medical devices being placed on the market in Great Britain.
Reference: UKCA marking and training - Gain market access in Great Britain | BSI (bsigroup.com)
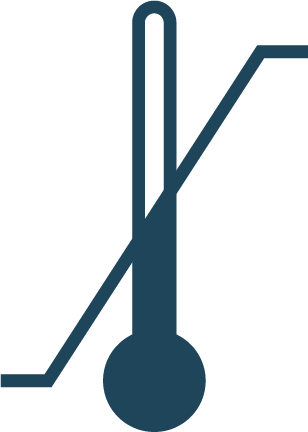
Upper & Lower Temperature Limits
Indicates the range of humidity which the medical device can be safely exposed to.
Reference: BS EN ISO 15223-1:2016 REF No: 5.3.7
View our full range of solutions
Take a look at our wide range of products across Infection Prevention, Clinical Waste Management, and Surgical Solutions
View More